Healthcare Utilisation and Seroprevalence Survey (HUTS)
About this project
The Healthcare Utilisation and Seroprevalence of COVID-19 (HUTS) study is a cross-sectional community survey being conducted in three communities serviced by healthcare facilities where severe respiratory illness (SRI) and influenza-like illness (ILI) surveillance is conducted in South Africa (Mitchell’s Plain, Pietermaritzburg and Klerksdorp) during and after the second wave of SARS-CoV-2 infections in South Africa. The study aims to explore the healthcare seeking behaviour for and cost of respiratory illness during the pandemic and to estimate SARS-CoV-2 community seroprevalence and the COVID-19 knowledge, attitudes and practices (KAP) of the selected communities. The study will complement data from inpatient and outpatient syndromic surveillance conducted in the same target communities to document the clinical spectrum of illness, including the proportion of asymptomatic, mild, severe and fatal cases, both medically and non-medically attended. Serology testing for SARS-CoV-2 is important in order to better quantify the number of COVID-19 cases, including those that may have been asymptomatic or recovered without having been tested. Public health action is guided by the incidence of infection, and therefore understanding the full burden of infection and potentially immunity, is important. Surveillance of antibody seropositivity in the South African population will allow inferences to be made about the extent of infection in the community.
Objectives
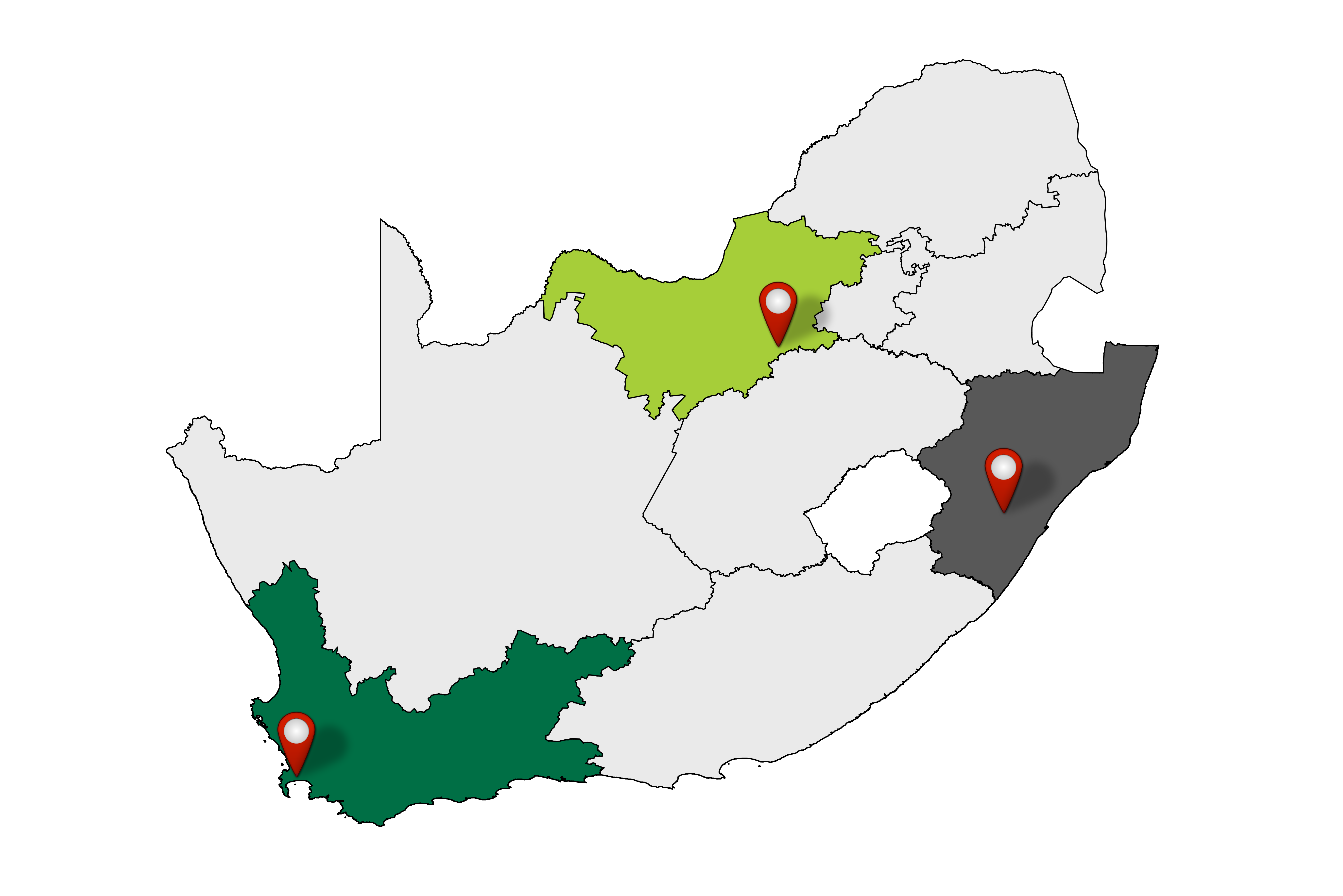
In three communities in South Africa, Klerksdorp North West Province, Pietermaritzburg KwaZulu-Natal and Mitchells Plain Western Cape, address the following:
Primary Objectives
- Characterize healthcare seeking behaviour for respiratory illness during the first wave of the COVID-19 pandemic
Determine the proportion of mild and severe respiratory illnesses that were not medically attended during the epidemic period - To estimate the household and community economic burden of respiratory illness during the first wave of the epidemic
- To describe the economic impact of mitigation measures on households during the first wave of the epidemic
- Describe knowledge, attitudes and practices related to COVID-19 in the community, including prevention
- Determine the seroprevalence of antibodies to SARS-CoV-2 following the first wave of the pandemic, by age group and HIV-infection status.
Secondary Objectives
- Provide a more accurate estimate of the burden of COVID-19 in the community by adjusting facility-based surveillance data for healthcare seeking behaviour
- Assess the sensitivity of sentinel surveillance for COVID-19 at each site
- Identify risk factors for SARS-CoV-2 infection including age, HIV infection and underlying illnesses
- To estimate the SARS-CoV-2 infection case-fatality ratio through triangulation with COVID sentinel surveillance and mortality data
- Determine the seroprevalence of antibodies to other respiratory viruses such as influenza, respiratory syncytial virus and other human coronaviruses
Impact
Understanding community healthcare utilization, KAP and economic burden on households associated with the COVID-19 pandemic is key to guide containment and mitigation measures in local settings and globally. In addition, understanding the disease burden of COVID-19 and groups at increased risk of severe COVID-19 is key to inform mitigation guidelines for ongoing and potential future epidemics.
Ethics
The study was approved by the University of the Witwatersrand Human Research Ethics Committee (Reference M200861).
Results
Gallery
Project Protocol
Funders and Collaborators
Sites